Oxygen
| Oxygen in the Periodic Table |
The Physical Properties of Oxygen
Qualitative - Oxygen in it’s original form has no colour, no odour, no taste and is in the state of gas. It has no texture, no lustre and is not malleable or ductile.
Quantitative - Also in it’s original form Oxygen is not hard, has an extremely low viscosity and is slightly soluble in water. It is denser than air and is a poor conductor. Oxygen changes from a gas to a liquid at the temperature of -182.96°C, liquid oxygen can frozen or made a solid at a temperature of -218.4°C.
The Chemical Properties of Oxygen
Oxygen is present in many compounds including water and carbon dioxide. Oxygen itself does not burn but it greatly supports combustion which is why fire’s need oxygen to keep burning. Oxygen itself does not react it water (it does form water though) but instead dissolves in it. | Oxygen is colourless, making it invisible to the human eye. |
Bohr-Rutherford Diagram
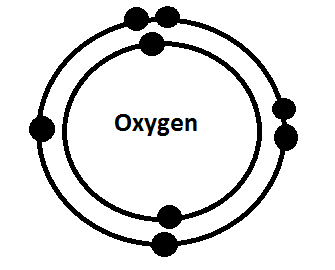
Why Group VI and Period 2?
Oxygen is located in group VI because it has 6 valence electrons and a 2- charge. It’s in the second period because it has 2 energy levels.
General Uses of Oxygen
General Uses of Oxygen

Why is it Important to the Periodic Table?
Oxygen is important to the Periodic Table because the physical Periodic Table would not be possible without it. This may sound a bit funny but think about it this way: Would any human be able to hold their breathe long enough to discover and test with elements that are inducted into today's Periodic Table?
| The History of Oxygen |
Oxygen can be used to form many useful compounds, these compounds can go from almighty water, to the rust that pretty much ruins your whole car. Oxygen is highly present in both ionic and molecular compounds. Some of the typical compounds formed by Oxygen are:
 |
| Dihydrogen Monoxide |
 |
| Carbon Monoxide |
| Iron (II) Oxide |
Oxygen Fun Facts
- Oxygen itself does not burn but instead supports the combustion of other substances.
-The Northern and Southern lights are caused by oxygen atoms.
-Oxygen is the third most abundant element in the universe.
-The most abundant element on Earth is Oxygen.

References
- All images came from Google Images.
- Information and examples of compounds: Common Compounds of Oxygen - O (EnvironmentalChemistry.com). N.p., n.d. Web. 12 Dec. 2016.
- Information on The History of Oxygen: "The Element Oxygen." It's Elemental - The Element Oxygen. N.p., n.d. Web. 12 Dec. 2016.
- Oxygen Fun Facts:Stewart, Dr. Doug. "Facts about Oxygen." Chemicool. N.p., n.d. Web. 12 Dec. 2016.
- Information and examples of compounds: Common Compounds of Oxygen - O (EnvironmentalChemistry.com). N.p., n.d. Web. 12 Dec. 2016.
- Information on The History of Oxygen: "The Element Oxygen." It's Elemental - The Element Oxygen. N.p., n.d. Web. 12 Dec. 2016.
- Oxygen Fun Facts:Stewart, Dr. Doug. "Facts about Oxygen." Chemicool. N.p., n.d. Web. 12 Dec. 2016.
No comments:
Post a Comment